
English articles
Why stakeholder analysis is essential for market access communication
Market access of pharmaceutical products in Germany is associated with numerous challenges, particularly around regulatory discussions. When the so-called Arzneimittelmarkt-Neuordnungsgesetz (AMNOG) was introduced in 2011, a significantly more rigorous benefit evaluation procedure was put into place. Since then it is more important than ever for pharmaceutical companies to strategically plan their market access communications and focus on stakeholder needs.
What does success look like?
It is important to truly understand and communicate the unmet needs of the relevant stakeholders that your new product could fulfil. The basis of such communications are analyses established early on and continued throughout the market access process.
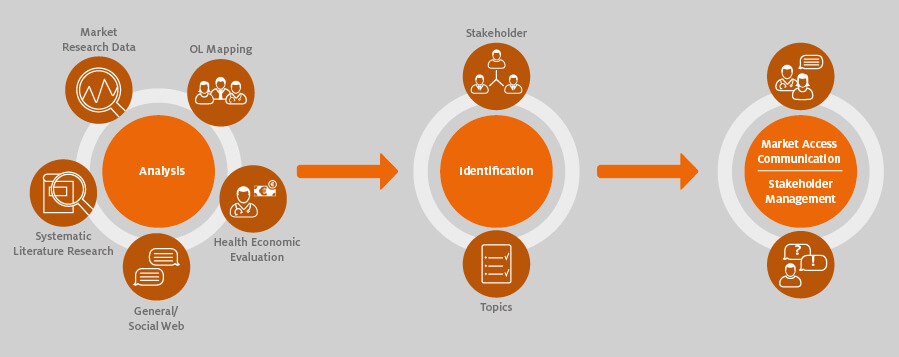
Opinion leader mapping, for example, is traditionally used to identify the key influencers and the potential “rising stars” among healthcare professionals, but it is also important to identify the other stakeholders, influencers and commentators in the area. The key point is to identify what matters most to each stakeholder group and map your product or “beyond the pill” offerings to each of them. The data generated by the analyses are used in the preparation of individual market access strategies for each stakeholder group.
In-depth discussions with select stakeholders can help to facilitate this. Dialogue with key representatives from each stakeholder group (including patients) will provide a rich source of insights into wider developments, trends and unmet needs that can help to shape your market access communications.
The multi-stakeholder analyses should begin several years (ideally two to three at the minimum) before the launch of a pharmaceutical product. Bear in mind that payers and regulatory institutes involved in the AMNOG process are important stakeholders to engage in dialogue about existing unmet needs and potential solutions. In addition, it is necessary in the AMNOG process to keep health service providers informed, for example, about the current status of the process.
Authors: Vanessa Wild (Team Market Access) & Ross Williams
Find more english articles on our english blog.

